What Comprehensive Oral Care Really Looks Like in Modern Den..
11 Min Read
ECMO uses technology from the cardiopulmonary bypass that allows gas exchange outside the body. Circulatory support can also be provided in addition to it. ECMO is especially useful for neonates with respiratory distress, although it is increasingly being used nowadays for adults too. Initially ECMO was not associated with good survival rates and was tried for adults with cardiac failure or ARDS (Adult Respiratory Distress Syndrome).
ECMO works by artificially removing the carbon dioxide from the body and the oxygenated red blood cells. Clinical Situations where ECMO can be started are:
ECMO has better survival and good outcomes in patients with cardiac arrest or cardiogenic shock.
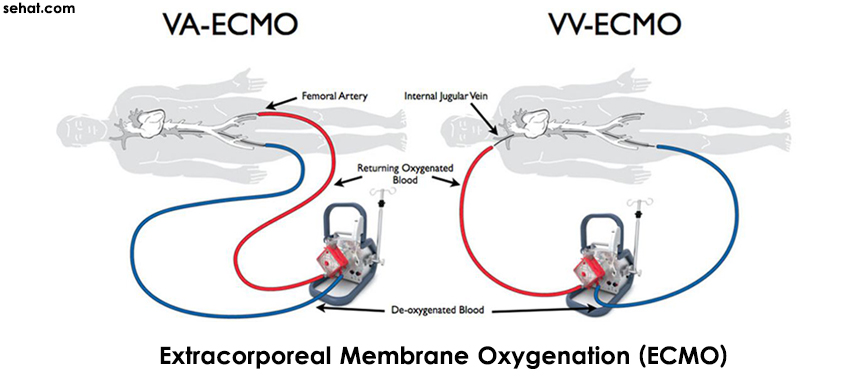
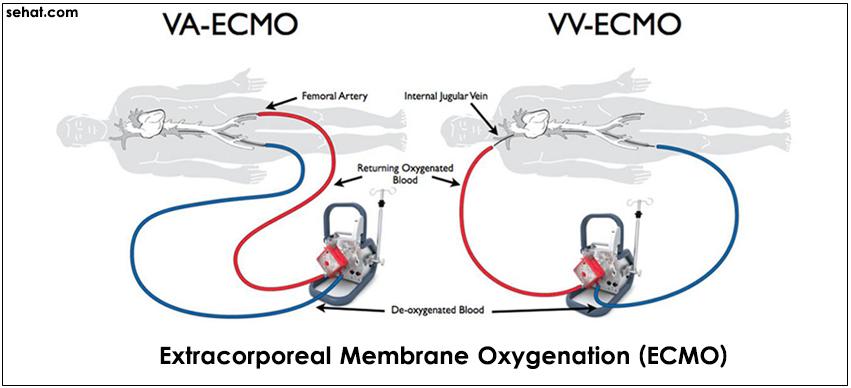
1) Veno-arterial ECMO (VA-ECMO): It allows gas exchange and haemodynamic support while blood is pumped from the venous to the arterial side
2) Veno-venous (VV-ECMO): It facilitates gas exchange; blood is removed from the venous side and then pumped back into it, but does not provide haemodynamic support
3) Arterio-venous ECMO (AV-ECMO): It facilitates gas exchange by using the patient’s own arterial pressure to pump the blood from the arterial to the venous side.
While ECMO is started, the goal is often to reduce the excess fluid that has accumulated in the extracellular space as a result of cardiac failure, sepsis or inflammation. Fluid restriction and diuretics may be effective, although many patients will require extracorporeal haemofiltration. This can often be performed on blood from the ECMO circuit.
Anticoagulation is critical to avoid the formation of clots in the ECMO circuit, while balancing the patient’s risk of bleeding. Thrombocytopenia is a common problem and regular platelets transfusions may be necessary to keep the platelet count over an arbitrary limit to decrease the risk of spontaneous bleeding. If heparin-induced thrombotic thrombocytopenia is diagnosed, the use of alternative anticoagulant is required. It is possible to run ECMO without heparin, but this increases substantially the thrombotic risk.
Patients with irreversible organ damage, multiorgan failure, and candidates rejected for transplantation are not usually not good candidates for ECMO. Some studies say ECMO should not be given in those who cannot be anticoagulated, as well as patients with aortic dissection or regurgitation do not benefit well from ECMO. It is always better to consult a ECMO specialist before initiating ECMO therapy.
1) Infections: Infective complications can be related to the indwelling lines, access sites, or primary pathology. Strict aseptic handling of lines is required.
2) Hemorrhage: Arterial cannulation is related to higher risk of bleeding. Haemorrhage can occur in any organ, with intracranial bleeding being the most devastating.
3) ECMO Failure: In some cases there can be circuit failure or breakage in the functioning of ECMO.
ECMO can be used in adult patients with cardiogenic shock or ECMO who fail to respond to conventional therapy. Although the evidence in favour of ECMO for ARDS treatment is not strong enough to make a general recommendation, it should be considered when other therapies fail.